
We're interested in the fascinating chemistry that occurs at electrochemical interfaces and plays a critical role in dictating performance of catalysts involved in energy conversion and storage. Combining the tools of materials science and surface physics, we use spectroscopic approaches to link the surface chemistry of catalysts to their electronic structure and performance. This mechanistic understanding can in turn guide the rational design of more active catalysts, increasing the efficiency of electrochemical devices (e.g. fuel cells, electrolyzers). We're particularly interested in the interface between earth-abundant catalysts, comprised of transition metal oxides, with water -- with further applications in water purification.
Electrochemistry can be used to store energy in chemical bonds, which can later be extracted upon demand. A prototypical example of this is splitting water into hydrogen and oxygen gas in an electrolyzer, driven by an applied voltage. We're working to develop catalysts that are earth abundant, efficient, and selective in driving the desired reaction. Projects include:
Seawater electrolysis (funded by NSF CAREER for fundamental studies, DOE WPTO for applied studies)
Molecular and Atomic EngineeRing of Interfacial Electro-catalytic Environments, MARIE (funded by DOE BES Science Foundations for the Energy Earthshots)

Electrochemistry can drive reactions in a distributed, scalable way. These processes can be used to upgrade low-value feedstocks to products with more desirable properties. We employ spectroscopic approaches to understand why certain catalysts are more efficient than others by understanding the reaction mechanism. We're also working to reduce the amount of precious metals required for these reactions (and in some cases, replace them entirely) by unique approaches in materials design. Reactions of interest include:
Electrochemical hydrogenation of organics (funded by DOE BES with PNNL)
Selective oxidation of methane to methanol (funded by ACS PRF)

Sustainable chemical manufacturing and circular processes can leverage electrochemistry to convert harmful waste products into benign or value-added streams. We're working to develop electrocatalysts that drive these reactions selectively and employ spectroscopy to understand and tailor the electrode/electrolyte interface. Projects include:
Electrochemical reduction of nitrate (funded by DOE BES Early Career)
Electrolysis-driven mineralization
Unique approaches
Some questions can only be answered digging deep into materials systems and simplifying an otherwise complex picture down to its critical parts.
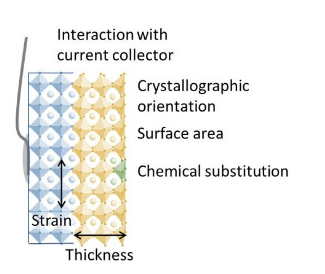
Intrinsic activity of oxide catalysts
Electrochemical studies of model systems
In order to rationally design more active catalysts for energy and conversion and storage, thus reducing material cost for commercial technologies, we study the fundamental processes that occur on model catalyst systems for oxygen reduction and evolution. Electrochemical studies of well-defined surfaces grown by pulsed laser deposition or molecular beam epitaxy establish the intrinsic activity of oxide catalysts in a way that cannot be realized with polydisperse nanoparticle systems, and can also reveal how different terminations and structures affect the kinetics. These studies of epitaxial thin films were among the first to probe phenomena that are not straightforward to isolate in nanoparticles, such as the role of oxide band structure, interfacial charge transfer (the “ligand” effect), strain, and crystallographic orientation.

Surface chemistry of oxide catalysts
Spectroscopic probing of surface species in situ
Ambient pressure X-ray photoelectron spectroscopy is a recent technique which can probe the surface species present in environments approaching that of material operation, bridging the pressure gap between surface science and application. We have used this approach to investigate the adsorption of water and other small molecules on well-defined thin film surfaces, quantifying the dependence of speciation on the chemical potential. The reactivity of the surface depends on both the transition metal of complex oxides and the termination in the ternary perovskite crystal structure. This fundamental insight brings molecular understanding to the wetting of oxide surfaces, as well as the role of hydrogen bonding in catalysis.